Introduction
Transformation of indolent lymphomas to diffuse large B-cell lymphoma (DLBCL) is associated with poor outcomes, particularly for patients (pts) with relapsed or refractory (r/r) disease. While chimeric antigen receptor (CAR) T cell therapy has revolutionized the management of r/r DLBCL and is approved for transformed indolent lymphoma (TIL), patients with TIL were largely underrepresented in the pivotal trials leading to the approval of CD19CAR T. In this study, we evaluate the safety and efficacy of standard of care (SOC) CD19CAR T in TIL relative to de novo DLBCL (dDLBCL).
Methods
We performed a multi-center retrospective study to evaluate safety and efficacy of SOC CD19CAR T in adults with r/r TIL and dDLBCL treated at 5 centers. Eligibility criteria included age ≥ 18 years old, diagnosis of DLBCL (de novo or transformed), r/r disease, and receipt of SOC CD19CAR T. Pts who received CAR T on clinical trials were excluded. We defined TIL as DLBCL transformed from follicular lymphoma (FL), marginal zone lymphoma (MZL), or Waldenstrom's macroglobulinemia (WM). Pts with Richter's syndrome were excluded. Safety endpoints: incidence and severity of cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS) per American Society for Transplantation and Cellular Therapy (ASTCT) consensus grading. Response was assessed using 2014 Lugano Criteria. Efficacy endpoints include best overall response rate (ORR) (proportion of patients achieving a complete or partial response), complete response (CR) rate, progression/relapse-free survival (PFS) and overall survival (OS).
Results
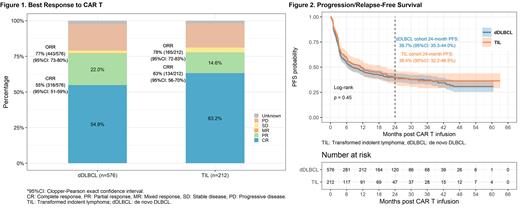
A total of 788 pts were included (212 (27%) TIL, 576 (73%) dDLBCL) with the CAR T infusion date ranging from 12/2017 to 03/2023. In the TIL cohort, 192 (91%) pts transformed from FL, 14 (7%) MZL, 5 (2%) WM, and 1 indolent lymphoma NOS. In TIL and dDLBCL cohorts, respectively, the median age at infusion was 64 (range 33-85)/64 (21-85) yrs, 37%/36% were female, pts received a median of 3 (range: 1-11)/ 3 (1-13) prior lines of therapy, and 11%/13% had ECOG ≥2 at time of lymphodepletion. In TIL cohort, 44 (21%) pts received prior stem cell transplantation (41 autologous, 3 allogeneic, and none both), while in dDLBCL cohort, 135 (23%) received prior SCT (122 autologous, 9 allogeneic, and 4 both). In TIL and dDLBCL cohorts, 76%/73% received axicabtagene ciloleucel, 17%/18% tisagenlecleucel, and 7%/10% lisocabtagene maraleucel, respectively.
Similar toxicities were observed in both cohorts. Any grade CRS/grade ≥ 3 CRS were observed in 76% (95% CI: 70-82%)/ 5% (3-9%) of TIL pts and 81% (78-84%)/ 6% (4-8%) of dDLBCL pts, respectively. Any grade ICANS/grade ≥ 3 ICANS rate were observed in 37% (95%CI: 31-44%)/ 16% (11-21%) of TIL pts and 49% (45-53%)/ 23% (20-27%) of dDLBCL pts, respectively. In TIL and dDLBCL cohorts, respectively, 49%/59% of pts received tocilizumab for CRS and 41%/51% received steroids for CRS or ICANS.
Efficacy outcomes were comparable. The best ORR/ CR rate were 78% (95%CI: 72-83%)/ 63% (56-70%) in the TIL cohort and 77% (73-80%)/ 55% (51-59%) in the dDLBCL cohort. Among responders, the median time from infusion to best response was 33 days (IQR: 29-69) and 34 days (IQR: 29-85), respectively. With a median overall follow-up of 13.4 (0.03-63.1) months (mons), the median PFS was 11.2 mons (95%CI: 6.3-20.8) in the TIL and 7.9 mons (95%CI: 6.1-12.2) in the dDLBCL cohort, with a 24-month PFS of 39.4% (95%CI: 32.2-46.5%) and 39.7% (95%CI: 35.5-44.0%) respectively (logrank p=0.45). Median OS was 41.7 mons (95%CI: 24.9-not reached) and 29.7 mons (95%CI: 22.1-not reached) with a 24-month OS of 57.8% (95%CI: 50.1-64.7%) and 53.2% (95%CI: 48.4-57.7%) in TIL and dDLBCL cohorts, respectively (logrank p=0.49). There was no statistically significant difference between the survival outcomes of the TIL and dDLBCL cohorts. 41% (86/212) and 42% (241/576) pts died in the TIL and dDLBCL cohorts respectively, with similar rates of CAR T cell related deaths (9.3% vs 7.1%).
Conclusion
This study represents the largest evaluation to date of outcomes of CD19CAR T in TIL. The results indicate that CD19CAR T is tolerable and active in pts with r/r TIL with safety and efficacy outcomes similar to that observed in pts with dDLBCL. Our findings suggest CD19CAR T cell therapy may be able to overcome the historically poor prognosis associated with r/r TIL.
Disclosures
Merryman:Abbvie: Membership on an entity's Board of Directors or advisory committees; Genentech/Roche: Research Funding; Seattle Genetics: Membership on an entity's Board of Directors or advisory committees; Intellia: Membership on an entity's Board of Directors or advisory committees; Merck: Research Funding; Epizyme: Membership on an entity's Board of Directors or advisory committees; Alphasights: Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees, Research Funding; Adaptive Biotechnology: Membership on an entity's Board of Directors or advisory committees; Genmab: Membership on an entity's Board of Directors or advisory committees, Research Funding. Voorhees:AstraZeneca: Research Funding; Incyte: Research Funding; Morphosys: Research Funding; Novartis: Consultancy; Recordati: Consultancy, Research Funding. Seshadri:Kite: Consultancy; BeiGene: Consultancy; Roche: Research Funding; Eli Lilly: Research Funding. Patel:Nurix: Research Funding; Loxo Oncology: Consultancy, Research Funding; CRISPR Therapeutics: Research Funding; Fate Therapeutics: Research Funding; Sunesis Pharmaceuticals: Research Funding; Pharmacyclics/Janssen: Consultancy, Research Funding; Epizyme: Consultancy, Research Funding; Curis, Inc: Research Funding; Caribou Biosciences: Consultancy; Kite: Consultancy, Research Funding, Speakers Bureau; Genentech/Roche: Consultancy, Research Funding; Trillium Therapeutics/Pfizer: Consultancy, Research Funding; TG Therapeutics: Consultancy, Speakers Bureau; Morphosys: Consultancy; Xencor: Consultancy, Research Funding; Merck: Consultancy, Research Funding; MEI Pharma: Consultancy, Research Funding; BeiGene: Consultancy; Bristol Myers Squibb: Consultancy, Research Funding, Speakers Bureau; AstraZeneca: Consultancy, Research Funding, Speakers Bureau; ADC Therapeutics: Consultancy; Adaptive Biotechnologies: Research Funding; Abbvie: Consultancy. Andreadis:pharmacyclics: Honoraria; Novartis: Research Funding; BMS: Honoraria, Research Funding; Gilead: Honoraria; Epizyme: Honoraria; Astra Zeneca: Honoraria; Roche: Research Funding; Lilly: Research Funding; Merck: Research Funding. Kittai:Eli Lilly: Consultancy; BeiGene: Consultancy, Research Funding, Speakers Bureau; AstraZeneca: Consultancy, Research Funding; Abbive: Consultancy; Janssen: Consultancy; KITE: Consultancy; BMS: Consultancy. Jacobson:Axis: Speakers Bureau; Clinical Care Options: Speakers Bureau; Precision BioSciences: Consultancy, Honoraria, Other: Travel support; Nkarta: Consultancy, Honoraria; Humanigen: Consultancy, Honoraria, Other: Travel support; AbbVie: Consultancy, Honoraria; Daiichi Sanko: Consultancy, Honoraria; ImmPACT Bio: Consultancy, Honoraria; Instil Bio: Consultancy, Honoraria; Bluebird Bio: Consultancy, Honoraria; Epizyme: Consultancy, Honoraria; Ispen: Consultancy, Honoraria; Lonza: Consultancy, Honoraria, Other: Travel support; Celgene: Consultancy, Honoraria, Other: Travel support; Novartis: Consultancy, Honoraria, Other: Travel support; Bristol Myers Squibb: Consultancy, Honoraria; Kite, A Gilead Company: Consultancy, Honoraria, Research Funding. Herrera:Caribou Biosciences: Consultancy; Adicet Bio: Consultancy; AbbVie: Consultancy; Merck: Consultancy, Research Funding; Allogene Therapeutics: Consultancy; Seattle Genetics: Consultancy, Research Funding; Genentech/Roche: Consultancy, Research Funding; ADC Therapeutics: Consultancy, Research Funding; Tubulis GmbH: Consultancy; BMS: Consultancy, Other: Travel/Accommodations/Expenses, Research Funding; Pfizer: Consultancy; Takeda: Consultancy; Genmab: Consultancy; AstraZeneca/MedImmune: Consultancy; Regeneron: Consultancy; Kite, a Gilead Company: Research Funding; Karyopharm Therapeutics: Consultancy; Gilead Sciences: Research Funding; AstraZeneca: Research Funding. Budde:Amgen: Research Funding; Roche: Consultancy; AstraZeneca: Consultancy, Research Funding; ADC Therapeutics: Consultancy; Merck: Research Funding; MustangBio: Research Funding; Novartis, Gilead, F. Hoffmann-La Roche Ltd, BeiGene, Genentech, Inc.: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal